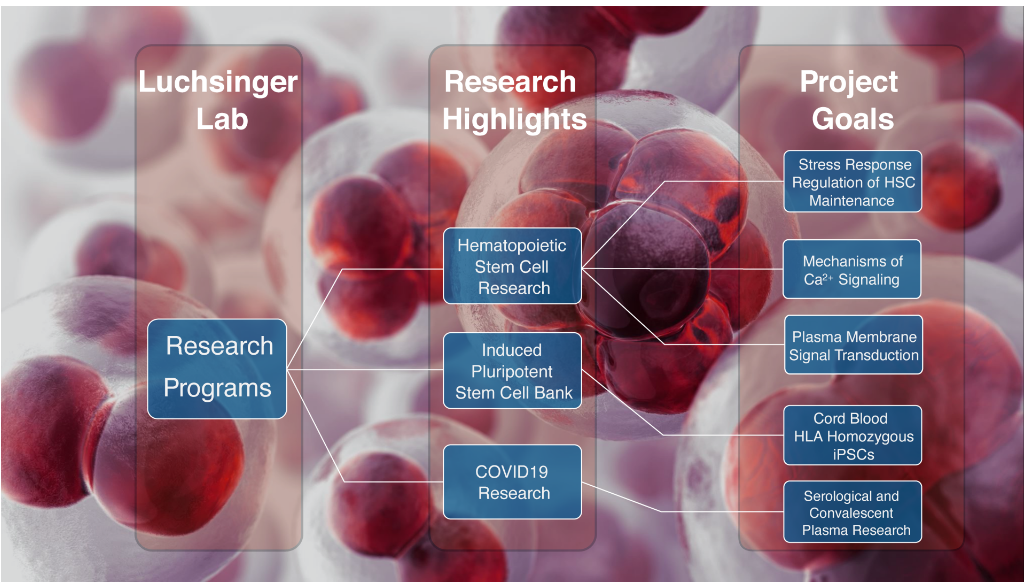
Research Areas
Hematopoietic Stem Cell Research
Hematopoietic stem cells (HSC), which reside in the bone marrow of adults, have the ability to replenish all cell types found in the blood over an entire lifetime. In a treatment of last resort, patients with hematological failure or malignancy currently receive transplantation of immunologically compatible bone marrow from healthy donors to regenerate the patient blood system with healthy cells. However, immunotolerance and donor accessibility make bone marrow transplants a high risk and potentially complicated treatment. The ability to expand and bank patient-specific HSCs for future transplantation would revolutionize the treatment of many hematological diseases, however in vitro expansion of clinically transplantable HSCs has not been achieved to date. Thus, understanding the mechanisms by which HSCs renew and give rise to multiple cell types would vastly accelerate this clinical goal.
Almost 200 genes have been implicated in the regulation of hematopoietic stem cell (HSC) function, yet from this information the events necessary to impart stem cells with self-renewal and multipotency remains elusive. The research in my lab focuses on investigating novel signal transduction machinery and pathways that have yet to be explored in HSC maintenance and self-renewal. As we learn more about the innate signaling pathways that occur in HSCs and how they integrate in self-renewal and multipotency, it is the ultimate goal of our lab to leverage this information to devise novel pharmacological molecules, culture techniques and expansion technologies that may successfully expand sufficient quantities of HSCs for clinically relevant applications.
Induced Pluripotent Stem Cell (iPSC) Bank
The ability to reprogram adult somatic cells into induced pluripotent stem cells (iPSCs) has inaugurated a practical conception of patient-specific cellular therapy. Through directed differentiation of iPSCs into terminally differentiated cells, the raw materials to regenerate, or even replace, patient tissues are theoretically possible. Yet the ability to consistently producing high quality and cost-effective iPSC lines for each patient in a timely manner remains a considerable challenge in the field. Alternatively, the use of donor iPSC cell lines makes quality control and reproducibility far more achievable. However, immunological tolerance and potentially recipient rejection of allogeneic iPSCs remains a considerable hurdle in using “off-the-shelf” iPSC derived cell types. Identification of donor cells that are homozygous for HLA genes, which participates in initiating non-self signaling to the immune system, can reduce the degree of matching necessary between donor iPSCs and patients.
The National Cord Blood Program (NCBP) of NYBC was established for the collection and storage of clinical-grade, HLA-typed cord blood (CB) units for transplantation therapies thus making NCBP CB units an attractive resource for deriving HLA homozygous iPSCs. Theoretically, with limited number of homozygous donors spanning a wide HLA cross-section of the population, a large percentage of the patients would be covered with potentially compatible iPSC lines and would advance the possibility of iPSC-derived therapies towards a reality. Research efforts in the iPSC lab focus on optimization of isolation, reprogramming and characterization of HLA-homozygous lines derived from CD34+ CB cells and establish the NYBC master bank of clinical-grade HLA homozygous iPSCs.
Research News
- Healy, M. (2020) ‘As the economy reopens, scientists still have a lot to learn about coronavirus immunity‘, Los Angeles Times, 12 June.
- Park, A. (2020) ‘Antibody Tests Don’t Mean a Ton Right Now. But That Could Change Soon‘, TIME, 16 June.
Funding Support
- NIH R01 HL155574 (PI Luchsinger) 07/2021 – 06/2026 “Hormetric ER stress Regulation of Hematopoietic Stem Cell Function”
Education and Training
Postdoctoral Institute
Columbia University Medical Center
Degree Institutions
PhD Biochemistry; Boston Univ. School of Medicine, Boston, MA
MA Chemistry; Boston University, Boston, MA
BS Chemistry; Marquette University, Milwaukee, WI
Larry Luchsinger, PhD Researcher Profile PDF
Publications
Shi PA, Luchsinger LL, Greally JM, Delaney CS. Umbilical cord blood: an undervalued and underutilized resource in allogeneic hematopoietic stem cell transplant and novel cell therapy applications. Curr Opin Hematol. 2022 Nov 1;29(6):317-326. doi: 10.1097/MOH.0000000000000732. Epub 2022 Aug 29. Review. PubMed PMID: 36066376; PubMed Central PMCID: PMC9547826.
Luchsinger LL. Hormetic endoplasmic reticulum stress in hematopoietic stem cells. Curr Opin Hematol. 2021 Nov 1;28(6):417-423. doi: 10.1097/MOH.0000000000000668. Review. PubMed PMID: 34232142; PubMed Central PMCID: PMC8544609.
Jain S, Garg K, Tran SM, Rask IL, Tarczon M, Nandi V, Kessler DA, Strauss D, Sachais BS, Yazdanbakhsh K, Rehmani S, Luchsinger L, Shi PA. Characteristics of coronavirus disease 19 convalescent plasma donors and donations in the New York metropolitan area. Transfusion. 2021 Aug;61(8):2374-2383. doi: 10.1111/trf.16421. Epub 2021 May 4. PubMed PMID: 33904609; PubMed Central PMCID: PMC8242807.
Jin DK, Nesbitt DJ, Yang J, Chen H, Horowitz J, Jones M, Vandergaast R, Carey T, Reiter S, Russell SJ, Kyratsous C, Hooper A, Hamilton J, Ferreira M, Deng S, Straus D, Baras A, Hillyer CD, Luchsinger LL. Seroprevalence of anti-SARS-CoV-2 antibodies in a cohort of New York City metro blood donors using multiple SARS-CoV-2 serological assays: Implications for controlling the epidemic and “Reopening”. PLoS One. 2021;16(4):e0250319. doi: 10.1371/journal.pone.0250319. eCollection 2021. PubMed PMID: 33909646; PubMed Central PMCID: PMC8081167.
Fidler TP, Xue C, Yalcinkaya M, Hardaway B, Abramowicz S, Xiao T, Liu W, Thomas DG, Hajebrahimi MA, Pircher J, Silvestre-Roig C, Kotini AG, Luchsinger LL, Wei Y, Westerterp M, Snoeck HW, Papapetrou EP, Schulz C, Massberg S, Soehnlein O, Ebert B, Levine RL, Reilly MP, Libby P, Wang N, Tall AR. The AIM2 inflammasome exacerbates atherosclerosis in clonal haematopoiesis. Nature. 2021 Apr;592(7853):296-301. doi: 10.1038/s41586-021-03341-5. Epub 2021 Mar 17. PubMed PMID: 33731931; PubMed Central PMCID: PMC8038646.
Luchsinger LL, Hillyer CD. Vaccine efficacy probable against COVID-19 variants. Science. 2021 Mar 12;371(6534):1116. doi: 10.1126/science.abg9461. PubMed PMID: 33707257.
Luchsinger LL, Ransegnola B, Jin D, Muecksch F, Weisblum Y, Bao W, George PJ, Rodriguez M, Tricoche N, Schmidt F, Gao C, Jawahar S, Pal M, Schnall E, Zhang H, Strauss D, Yazdanbakhsh K, Hillyer CD, Bieniasz PD, Hatziioannou T. Serological Assays Estimate Highly Variable SARS-CoV-2 Neutralizing Antibody Activity in Recovered COVID19 Patients. J Clin Microbiol. 2020 Sep 11:JCM.02005-20. doi: 10.1128/JCM.02005-20.
Ragnesola B, Jin D, Lamb CC, Shaz BH, Hillyer CD, Luchsinger LL. COVID19 antibody detection using lateral flow assay tests in a cohort of convalescent plasma donors. BMC Res Notes. 2020 Aug 6;13(1):372. doi: 10.1186/s13104-020-05212-0.PMID: 32762746
Luchsinger LL, Strikoudis A, Danzl NM, Bush EC, Finlayson MO, Satwani P, Sykes M, Yazawa M, Snoeck HW. Harnessing Hematopoietic Stem Cell Low Intracellular Calcium Improves Their Maintenance In Vitro. Cell Stem Cell. 2019 Aug 1;25(2):225-240.e7. doi: 10.1016/j.stem.2019.05.002. Epub 2019 Jun 6.PMID: 31178255
Corrigan DJ, Luchsinger LL, Justino de Almeida M, Williams LJ, Strikoudis A, Snoeck HW. PRDM16 isoforms differentially regulate normal and leukemic hematopoiesis and inflammatory gene signature. J Clin Invest. 2018 Aug 1;128(8):3250-3264. doi: 10.1172/JCI99862. Epub 2018 Jul 23. PubMed PMID: 29878897; PubMed Central PMCID: PMC6063481.
de Almeida MJ, Luchsinger LL, Corrigan DJ, Williams LJ, Snoeck HW. Dye-Independent Methods Reveal Elevated Mitochondrial Mass in Hematopoietic Stem Cells. Cell Stem Cell. 2017 Dec 7;21(6):725-729.e4. doi: 10.1016/j.stem.2017.11.002. Epub 2017 Nov 30. PubMed PMID: 29198942; PubMed Central PMCID: PMC5728653.
Chen X, Deng H, Churchill MJ, Luchsinger LL, Du X, Chu TH, Friedman RA, Middelhoff M, Ding H, Tailor YH, Wang ALE, Liu H, Niu Z, Wang H, Jiang Z, Renders S, Ho SH, Shah SV, Tishchenko P, Chang W, Swayne TC, Munteanu L, Califano A, Takahashi R, Nagar KK, Renz BW, Worthley DL, Westphalen CB, Hayakawa Y, Asfaha S, Borot F, Lin CS, Snoeck HW, Mukherjee S, Wang TC. Bone Marrow Myeloid Cells Regulate Myeloid-Biased Hematopoietic Stem Cells via a Histamine-Dependent Feedback Loop. Cell Stem Cell. 2017 Dec 7;21(6):747-760.e7. doi: 10.1016/j.stem.2017.11.003. Epub 2017 Nov 30. PubMed PMID: 29198940; PubMed Central PMCID: PMC5975960.
Adams WC, Chen YH, Kratchmarov R, Yen B, Nish SA, Lin WW, Rothman NJ, Luchsinger LL, Klein U, Busslinger M, Rathmell JC, Snoeck HW, Reiner SL. Anabolism-Associated Mitochondrial Stasis Driving Lymphocyte Differentiation over Self-Renewal. Cell Rep.2016 Dec 20;17(12):3142-3152. doi: 10.1016/j.celrep.2016.11.065. PubMed PMID: 28009285; PubMed Central PMCID: PMC5189677.
Luchsinger LL, de Almeida MJ, Corrigan DJ, Mumau M, Snoeck HW. Mitofusin 2 maintains haematopoietic stem cells with extensive lymphoid potential. Nature. 2016 Jan 28;529(7587):528-31. doi: 10.1038/nature16500. Epub 2016 Jan 20. PubMed PMID: 26789249; PubMed Central PMCID: PMC5106870.
Kamezaki K, Luchsinger LL, Snoeck HW. Differential requirement for wild-type Flt3 in leukemia initiation among mouse models of human leukemia. Exp Hematol. 2014 Mar;42(3):192-203.e1. doi: 10.1016/j.exphem.2013.11.008. Epub 2013 Nov 20. PubMed PMID: 24269847; PubMed Central PMCID: PMC3959278.
Luchsinger LL, Patenaude CA, Smith BD, Layne MD. Myocardin-related transcription factor-A complexes activate type I collagen expression in lung fibroblasts. J Biol Chem.2011 Dec 23;286(51):44116-25. doi: 10.1074/jbc.M111.276931. Epub 2011 Nov 2. PubMed PMID: 22049076; PubMed Central PMCID: PMC3243519.
Xu Y, Luchsinger L, Lucey EC, Smith BD. The effect of class II transactivator mutations on bleomycin-induced lung inflammation and fibrosis. Am J Respir Cell Mol Biol. 2011 Jun;44(6):898-905. doi: 10.1165/rcmb.2009-0416OC. Epub 2010 Aug 12. PubMed PMID: 20705943; PubMed Central PMCID: PMC3135849.
Wu X, Kong X, Luchsinger L, Smith BD, Xu Y. Regulating the activity of class II transactivator by posttranslational modifications: exploring the possibilities. Mol Cell Biol. 2009 Nov;29(21):5639-44. doi: 10.1128/MCB.00661-09. Epub 2009 Aug 31. Review. PubMed PMID: 19720744; PubMed Central PMCID: PMC2772741.